Chemistry of Lead in Drinking Water Distribution Systems
Lead is an element known since antiquity whose Latin name is synonomous with plumbing. In the earth's crust, it is present in minor amounts, about 20 mg/kg, but is easy to extract. It commonly is accompanied by zinc. In addition to plumbing components, it had been widely used in paint and as a gasoline additive, which has led to extensive soil contamination. It is now known to be toxic in even minuscule amounts, so vigorous efforts have been made to remove these sources of exposure. Its toxicity stems in part form its similarity to Ca. The 2+ oxidation state of Pb is about the same size as the Ca ion, which is also 2+. Accordingly, if Pb is ingested, it can bind irreversibly to enzyme sites occupied by Ca and it can enter the bones, where it remains as a stubborn toxic reservoir.
The aqueous chemistry of lead is controlled by both acid-base reactions (exchange of protons) and oxidation-reduction reactions (exchange of electrons). It has three common oxidation states: 0, +2, and +4, although the +2 is by far the most common. Metallic lead is actually not stable in the presence of water at ordinary temperatures. Instead it oxidizes rather quickly, and develops a thin coating of PbO, either as the mineral litharge or the mineral massicot. In distribution systems with moderate to high alkalinity water, one of the carbonates (cerussite, hydrocerussite or plumbonacrite) eventually replaces these oxides. Because they are much less soluble, this transformation lowers dissolved lead in the system. Ih the following table, the solubilities of various Cu, Zn, and Pb minerals commonly found in distribution systems are compared. Equilibrium is calculated with respect to average composition of treated water in a large midwestern utility using a surface water source with an alkalinity of 76 mg/L and phosphate at 0.36 mg/L as P.
| Relative solubility of scale minerals | ||||
| Phosphate | Carbonate | Sulfate | Oxide(2+) | |
| Copper | 1.52 | 0.022 | 0.48 | 0.082 |
| Zinc | 3.86 | 386 | 314264 | 4.07 |
| Lead | 0.00016 | 0.051 | 42.4 | 143 |
| Concentrations in mg/L of total dissolved metal | ||||
Note there is no consistent trend with either the metal or the anion: Pb is least soluble as the phosphate, Cu as the carbonate. Zinc compounds, however, tend to be much more soluble than the other two metals.
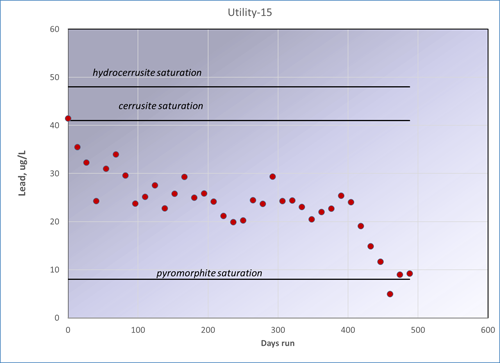
For systems with a high chlorine residual, the Pb4+ mineral, plattnerite becomes important, and it has an even lower solubility. Finally, if phosphate is added at the treatment plant, very insoluble Ca-Pb phosphate minerals can form, which will bring down Pb levels as well. This process of conversion of an early metastable scale to a later stable one takes months to a few years for lead minerals. Copper tends to be slower.
Approach to equilibrium for a fresh lead surface, Utility 15, over a period of a year and a half.